-40%
Handheld Lung Spirometer ,PC Software,Pulmonary Function,Lung Volume CMS-SP10
$ 99.79
- Description
- Size Guide
Description
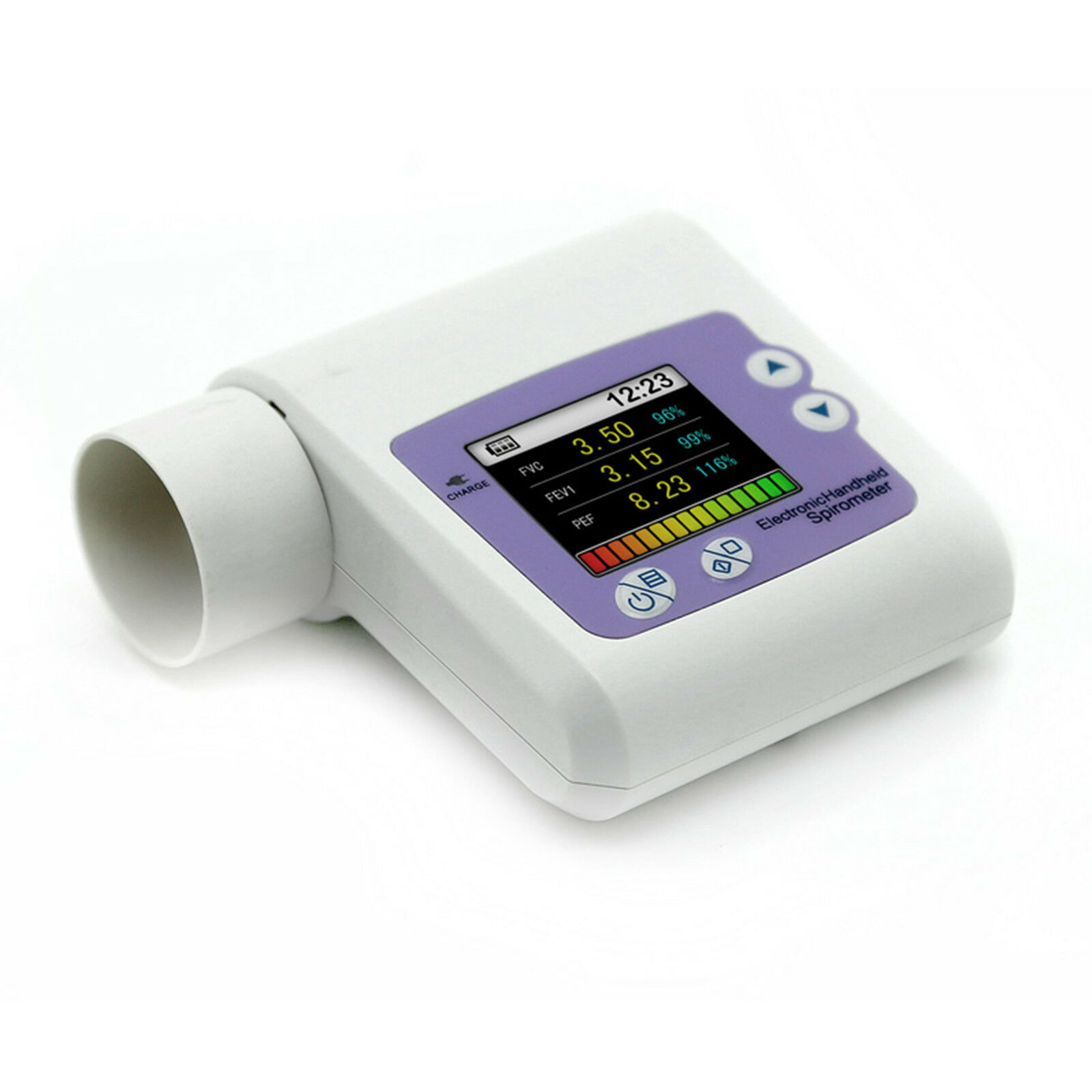
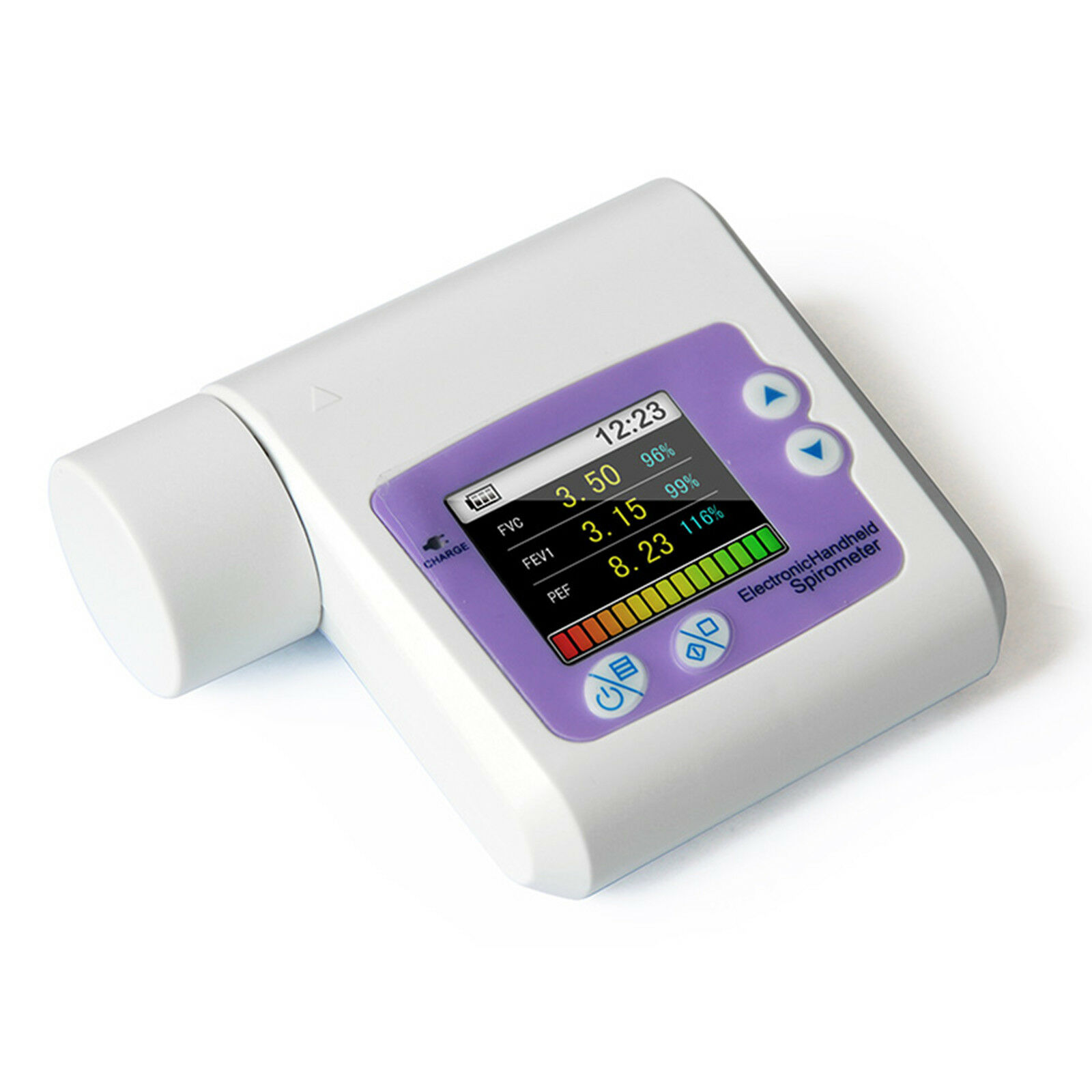
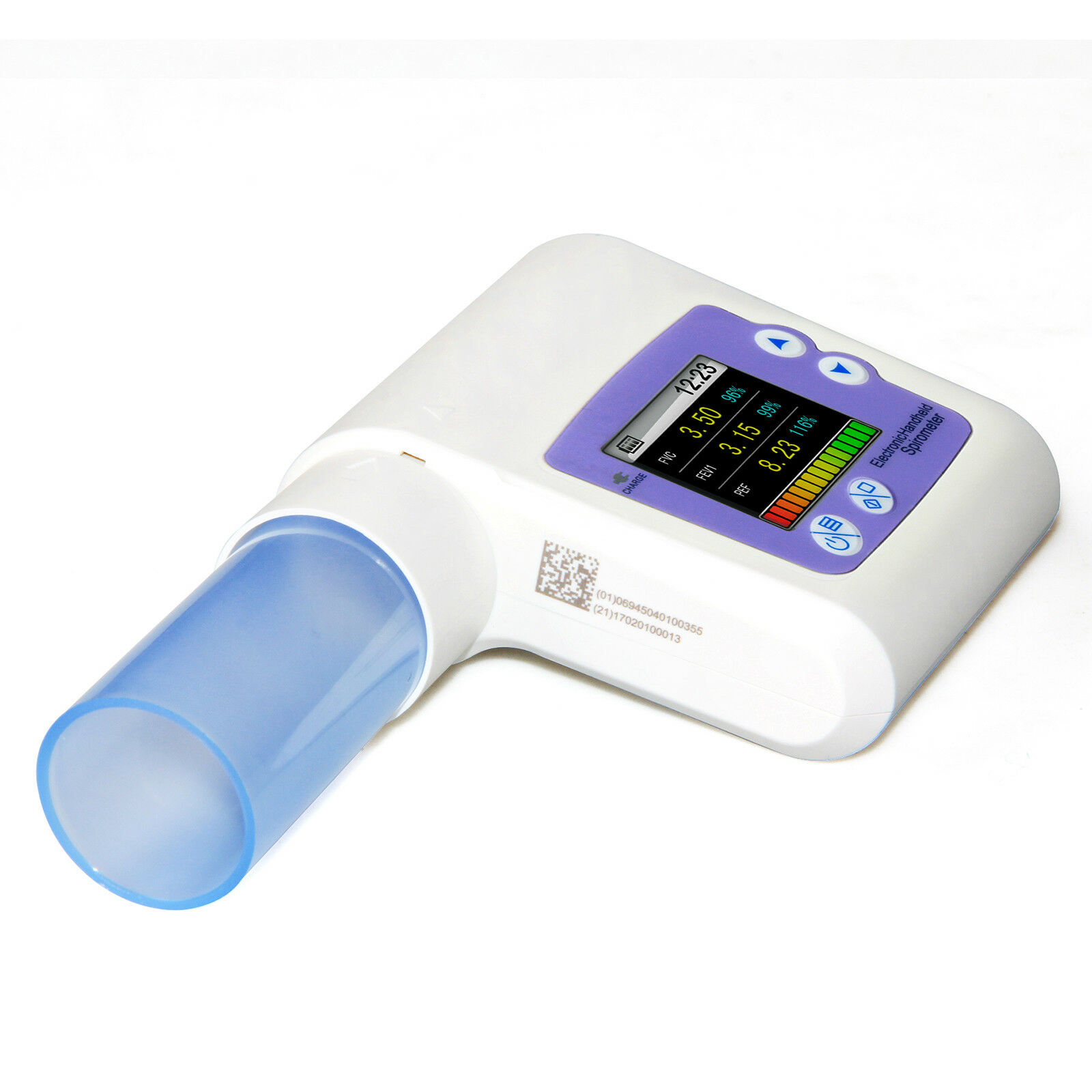
Handheld Lung Spirometer ,PC Software,Pulmonary Function,Lung Volume CMS-SP10Introduction:
SPIROMETER is a hand-held equipment for checking lung conditions, adopts the infrared mode for measuring relative items, and is applicable for hospital, clinique for routine test.
Features:
Forced Vital Capacity (FVC), Forced Expired Volume in one second (FEV1), the ratio of FEV1 and FVC (FEV1%), Peak expiratory flow (PEF), 25% flow of the FVC (FEF25), 75% flow of the FVC(FEF75) and average flow between 25% and 75% of the FVC(FEF2575) can be measured. Besides, the testee condition can be shown by the ratio of the measured value and the predicted value.
Flow rate-volume chart, volume-time chart display.
Data memory,delete, upload and review.
Trend chart display.
Scaling(Calibration).
Information prompts when volume or flow goes beyond the limits.
Automatic power off when there is no operation in one minute.
Rechargeable lithium battery and with charging tips.
Battery power display.
Performance:
Display mode: 1.8'' color LCD
Display resolution: 160*128
Volume range: 0~10L
Volume accuracy: ±3% or 0.05L (whichever is greater)
Flow range: 1 L/S ~16L/S
Flow accuracy: ±10% or ±0.3L/s(whichever is greater)
Working current: 60 mA
Power supply: DC3.7V rechargeable lithium battery
Safety classification: internally powered equipment, type BF applied part
Accessories:
An User Manual
An USB data line
A mouthpiece
A power adapter
A CD (PC software)
A nose clip(optional)
Physical characteristic:
Dimension: 97mm(L)× 89mm(W)×36mm(H)
Weight: 150g
Buy safe Products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for
REFERENCE:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state
and local regulatory agencies.If the item is subject to FDA regulation, We will verify your status as an
authorized purchaser of this item before shipping of the item.
If you have questions about legal obligations regarding sales of medical devices, you should consult with
the FDA's Center for Devices and Radiological Health.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with
the code 197923,
and certified by FDA of United States and CE,TUV of Europe.The Fingertip Pulse
Oximeter that is FDA 510K Approved
Only English user guide,if you need any other language,please contact us!
Track Page Views With
Auctiva's FREE Counter