-40%
Sleep Apnea Meter Respiratory Monitor SpO2 Nose Air flow Software Analyzer Alarm
$ 52.27
- Description
- Size Guide
Description
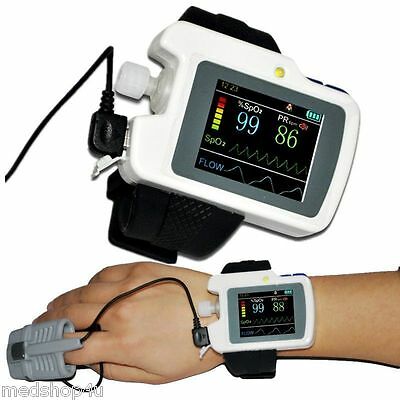
Sleep apnea screen meter RS01, SPO2 Pulse Rate Air Flow Monitor, Alarm+SoftwareI
ntroduction
The RS01 device is suitable for the one who suffers with such diseases as SAHS (sleep apnea-hypopnea syndrome), Its functions including patient’s sleep apnea monitoring and SpO2 monitoring, which can be applied in hospital or at home.
Features
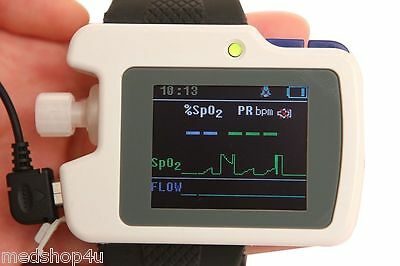
1)SpO2 and PR value display, pulse sound and bar graph indication.
2)Pulse waveform and nose flow waveform display
3)Alarms for low power, finger out and exceeding limits
4)Battery status indication
5)Real-time clock
6)Timing power on/off
7)Storage of multiple cases
8)Continuous 12 hours data record
9)Uploading data via USB port
10)PC analysis software
Performance
Nose air flow measurement range: 0 rpm~40 rpm, accuracy: ±2 rpm.
SpO2 measurement range: 0%~100% (resolution 1%), accuracy: 70%~100%, ±2; less than 70%, unspecified.
PR measurement range: 30 bpm~250 bpm, accuracy: ±2 or ±2% (whichever is greater).
Resistance to surrounding light: The deviation between the value measured in the condition of man-made light or indoor natural light and that of darkroom is less than ±1%.
Power consumption: ≤100 mA.
Power supply: 3.7 V rechargeable lithium battery.
Safety type: internally powered equipment, type BF applied part.
Accessories
A power adapter
A USB data line
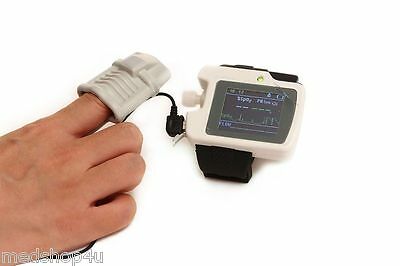
A Nasal cannula
Two kinds of SpO2 probes
A CD (PC software)
A User Manual
Physical characteristic
Dimension: 69 mm (L)×50mm (W) ×17.3 mm (H)
Weight: about 100g
Working environment:
Temperature: 10 ℃ ~ 40 ℃
Relative humidity: ≤75 %
Atmospheric pressure: 700 hPa~1060 hPa
Storage environment:
Temperature: -40 ℃ ~ +55 ℃
Relative humidity: ≤95 %
Atmospheric pressure: 500 hPa~1060 hPa
Buy safe products
The following FDA Disclaimer is required for all eBay listing in Healthcare category and is included for REFERENCE: The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If the item is subject to FDA regulation, we will verify your status as an authorized purchaser of this item before shipping of the item.
The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE,TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved
International Buyer--Please note:
Import duties,Taxes are not included in the item price.These charges are buyers' responsibility
We are the manufacturer of medical equipment for 17 years, the following are our main products:
ECG / ECG holter EEG / EEG Holter B-ultrasound
Patient Monitor Fetal doppler Fetal Monitor
Spo2 Monitor Stethoscope Medical Image....
Agent in the world!
We are look for partners in the world now!
Do you want to become our agent in you conutry?
Join US!
Skype:xuebox3
contec.med.mary at hotmail.com
Only English User Manual,Please contact us if you need other language version.thank you